New publication with insights into how to control cell adhesion dynamics and aid in designing new biomaterials for medical use
Apr 11, 2024
Julie Buisson et al. have just published a paper at Nano Letters (ACS Publications) on a new reverse mechanotransduction pathway by chromatin remodeling that influences the cell adhesion strength from the nucleus to the cell surface.
Chromatin remodeling is a key component in the regulation of cellular functions. Cells are subjected to physical cues from their extracellular environment (e.g., tissue or new implants), which in turn alters the organization of their chromatin.
This mechanotransduction process involves a successive transmission of forces by different molecular connectors, going from the cell surface to the nucleus containing chromatin.
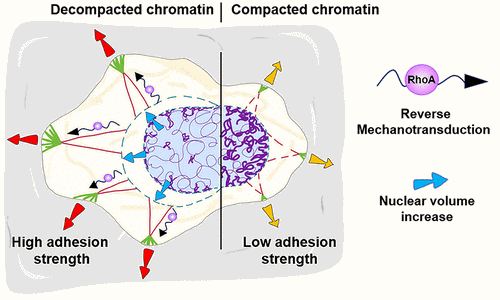
The team of the BioTUNE's Beneficiary Biomaterials & Bioengineering in Strasbourg (INSERM U1121, France) has explored a new way of mechanical signal transmission: reverse mechanotransduction. By using a microscope FluidFM to measure cell adhesion strength on a substrate, Buisson et al. found that artificially compacting the chromatin makes cells smaller and weaker at sticking to the surface while inducing chromatin decompaction restores their size and adhesion strength. Interestingly, cells can recycle broken-down components to rebuild connections quickly.
These results indicate a new reverse mechanotransduction pathway by chromatin remodeling that influences the cell adhesion strength from the nucleus to the cell surface.
Understanding this mechanism could offer insights into how to control cell adhesion dynamics and aid in designing new biomaterials for medical use.
The full information on this paper is:
J. Buisson, X. Zhang, T. Zambelli, P. Lavalle, D. Vautier, M. Rabineau. Reverse Mechanotransduction: Driving Chromatin Compaction to Decompaction Increases Cell Adhesion Strength and Contractility. Nano Lett. 2024, 24, 14, 4279–4290. doi: https://doi.org/10.1021/acs.nanolett.4c00732
Share: